Project Description

Description
-
Brand:乐动体育最新版本
-
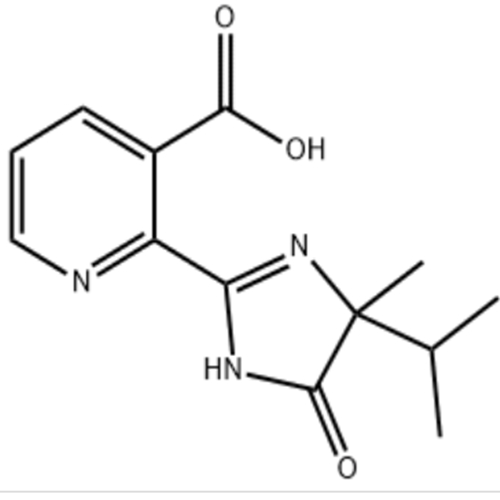
产品名称:Imazapyr
-
Synonyms:AC252925;CL252925;2-(4-Methyl-5-oxo-4-propan-2-yl-1H-imidazol-2-yl)pyridine-3-carboxylicacid;Arsenal250A;Charper;2-(5-Oxo-4-methyl-4-isopropyl-1H-imidazole-2-yl)pyridine-3-carboxylicacid;AC-243997;ImazapyrStandard
-
CAS No:81334-34-1
-
MF:C13H15N3O3
-
MW:261.28
-
Packaging:25 kg/fiber drum,or according to the requirements of the customers
What is the use ofImazapyr acid
1. Imidazolinone herbicides.
Its mechanism of action is to inhibit the synthesis of branched chain amino acids. This product is a selective herbicide used for weeding in railways, highways, factories, warehouses, canals and forestry. The dosage is 500~2000g/hm2. It can control most annual and perennial herbs and macrophytes. It can be used for soil treatment and post-emergence. Stem and leaf treatment,乐动体育最新版本萌发后治疗更有效。
When treating stems and leaves, add 0.25% non-ionic surfactant to the solution. After 2 to 4 weeks, the herb plants will become chlorosis and tissues will deteriorate. The young leaves of the trees will turn red or brown within one month. All leaves fell within 3 months and eventually died.
2.这是一个新的broad-spectrum herbicide. It has excellent herbicidal activity against Cyperaceae weeds, annual and perennial monocotyledonous weeds and broad-leaved weeds after emergence; imidazolinone herbicides.
Its mechanism of action is to inhibit the synthesis of branched chain amino acids.
Imazapyr acid ofSpecification
| Specification or Properties: | |
| Items | Specification |
| Melting point | 169-173°C |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| density | 1.1923 (rough estimate) |
| refractive index | 1.5600 (estimate) |
| Appearance | White solid |
| Chemical nature | Appearance is white solid. m.p.169~173℃. 45℃ can be stable for 3 months, room temperature can be stable for 2 years. It is stable in pH 5-9, dark place and water medium. The half-life of hydrolysis in sunlight is 6 days (pH 5-9), and the half-life in soil is 3 to 4 months. It is corrosive and cannot be mixed and stored in unlined containers. Reacts with acids, bases and strong oxidants. |
| Toxicity | The original drug has acute oral LD50>5000mg/kg for rats, acute oral LD50>2000mg/kg for mice, and acute skin LD50>2000mg/kg for rabbits. It is moderately irritating to rabbit skin and irritating to eyes, but it can be recovered. Rainbow trout, bluegill fish LC50>100mg/L (96h), Daphnia LC50>100mg/L (48h). Quail and wild duck acute oral LD50>2150mg/kg, LC50>5000mg/kg feed (8d). The bee exposure LD50>0.1mg/head. This product is isopropylamine salt for rats with acute oral LD50>10000mg/kg, mice with acute oral LD50>10000mg/kg, mice with acute skin LD50>2000mg/kg. |
| Characteristic | Mazapyr is an organic heterocyclic herbicide, which is a biocidal herbicide. Its isopropylamine salt can be used before or after germination. It can be quickly absorbed by plant roots and leaves, inhibiting the biosynthesis of plant side chain amino acids and preventing The growth of weeds promotes its death. The control objects include all weeds, and have good herbicidal activity on Cyperaceae weeds, annual and perennial monocotyledonous weeds,PAMbroad-leaved weeds and weeds. It is suitable for non-arable land, rubber plantation, oil palm, forest and tea plantation. |
| Production method 1 | To synthesize diethyl ketonedioate, add 250 mL of ether and 48.0 g of sodium ethoxide. Cool to 4~6℃. A mixed solution of 68.8 g of diethyl oxalate and 49.7 g of ethyl acetate was added dropwise. Stir for 0.5h. Raise the temperature to 38-40°C. Reflux for 1h. Let stand overnight. Adjust the pH to 1-2 with 10% dilute sulfuric acid. Separate the ether layer. Wash with water. After the ether was evaporated from the ether layer, the residue was distilled under reduced pressure to collect 39.3 g of the 130-132°C/3200Pa fraction. Synthesis of diethyl 2-aminosuccinate Add 89.5 g of diethyl ketone dicarboxylate and 150 g of toluene. Heat and stir to 100~110℃. Then the ammonia gas was introduced for 8 hours. cool down. Wash the reaction solution with water. Toluene was removed under reduced pressure.乐动体育最新版本Distill under reduced pressure to collect 70.15g of 110~114℃/666.7Pa fraction. Synthesis of 5-ethylpyridine-2,3-carboxylic acid diethyl ester 30.58g of 2-ethylacrolein, 59.18g of diethyl 2-aminosuccinate, 2.7g of p-toluenesulfonic acid and 250g of DMF were added. React at 90°C for 5h. DMF was evaporated under reduced pressure. Add 500 g of toluene to the residue. Wash with 5% sodium hydroxide. The toluene was evaporated under reduced pressure. The residue was distilled under reduced pressure. Collect 70.5g of 148~150℃/266.6Pa fraction. 5-ethylpyridine-2,3-carboxylic acid anhydride was synthesized by adding 111g of 25% sodium hydroxide solution. Stir and heat to 55°C. 56.2 g of 5-ethylpyridine-2,3-carboxylic acid diethyl ester was added dropwise. The dripping time is about 20min. Reheat to 65°C for 15 minutes. Ethanol-water was evaporated under reduced pressure. Then 70 g water and 318 g tetrahydrofuran were added. In this case, the temperature is maintained at 40°C. The pH of the reaction solution was adjusted to 1.65 with 50% sulfuric acid. Separate the water phase. The oil phase is evaporated to remove tetrahydrofuran. Add 50.4 g of acetic anhydride and 19.1 g of 4-picoline. Stir at room temperature for 1 h. Distill under reduced pressure to remove low boiling point. 36.0 g of 5-ethylpyridine-2,3-carboxylic acid anhydride (content 81.52%) was obtained. Synthesis of imidazole nicotinic acid 7.31g 5-ethylpyridine-2,3-carboxylic acid anhydride, 5.2g 2-amino-2,3-dimethylbutanamide and 100 mL of toluene were added. Stir at room temperature overnight. 2.02g triethylamine (0.02mol) was added. Reflux dehydration. The toluene was removed under reduced pressure. The residue was added to 100 mL of aqueous solution. Adjust the pH to 9 with 10% sodium hydroxide. Separate the oil phase. The pH of the aqueous phase was adjusted to 3 with hydrochloric acid. The precipitated solid is recrystallized with a mixed solvent. 7.3 g of a colorless solid product was obtained. m.p.168~170℃. |
| Production method 2 | Dipicolinic acid loses water to produce dipic acid anhydride, and then reacts with (CH3)2CHCCH3(CN)NH2 to produce carbamoyl nicotinic acid, which is then hydrolyzed with hydrogen peroxide in an aqueous solution of sodium hydroxide, and cyclized at elevated temperature to synthesize imidazole niacin. |
What are the safety issues ofImazapyr acid
| Hazard Codes | Xi |
| Risk Statements | 36-52/53 |
| Safety instructions | 26-61 |
| WGK Germany | 2 |
| RTECS | US5682500 |
Imazapyr acid of raw materials
Ethyl acetate–>Tetrahydrofuran–>Diethyl oxalate–>Sodium ethoxide–>4-methylpyridine–>3-Methoxypropionic acid–>Diethyl oxaloacetate–>Butane Diethyl diacid–>Aspartic acid–>2-Ethacrolein–>2-Amino-2,3-dimethylbutyronitrile
Product Storage
Keep undercool, dry and well ventilated warehouses, guard against fire.
Related Products
Our Vision: Become the world’s first class chemical material supplier!
BaiFuChem is a professional chemicals supplier.If you are interested in our products or want to purchase from China, please send E-mail to sales@baifuchem.com.